Introduction: Understanding CO₂ Cryotherapy and Inflammation
Inflammation is a fundamental biological defense, yet when unchecked, it can lead to chronic disease and tissue damage. CO₂ cryotherapy has emerged as a groundbreaking approach to managing inflammation by delivering precise, localized cold using pressurized carbon dioxide gas. Unlike traditional cooling methods, CO₂ cryotherapy triggers rapid heat exchange at the skin’s surface, initiating controlled vasoconstriction, immune modulation, and neurochemical shifts that influence key inflammatory pathways. This non-invasive technique can reduce cytokine activity, limit edema, and support faster tissue recovery. The ability to target inflammation at the molecular level positions CO₂ cryotherapy as a powerful tool in pain management and rehabilitation. As our understanding of cryobiological responses deepens, so does the potential for integrating CO₂-based therapies across various clinical applications. This analysis explores the molecular mechanisms linking cold exposure to immune regulation, offering insights into how CO₂ cryotherapy represents a new frontier in inflammation control and tissue healing.
How CO₂ Cryotherapy Works on a Cellular Level
The therapeutic efficacy of CO₂ cryotherapy stems from its ability to create controlled thermal shock at the cellular level, initiating a cascade of beneficial physiological responses. Understanding these mechanisms requires examining both the physical properties of the technology and the biological responses it triggers within treated tissues.
Principles of CO₂ Cryotherapy Technology
CO₂ cryotherapy operates through the controlled application of pressurized carbon dioxide gas, which undergoes rapid expansion and cooling when released from specialized delivery systems. This process creates therapeutic temperatures reaching -78°C (-108°F), significantly colder than traditional ice therapy. The delivery mechanism typically involves precision applicators that can target specific anatomical regions with remarkable accuracy, ensuring optimal therapeutic exposure while minimizing peripheral tissue effects. Modern systems incorporate real-time temperature monitoring and pressure regulation to maintain consistent therapeutic parameters throughout treatment sessions, which typically last 10-15 seconds per application site.
Key Biophysical Effects of CO₂ Cold Exposure
The immediate biophysical response to CO₂ cryotherapy involves rapid vasoconstriction, altered cellular membrane permeability, and modified enzymatic activity within treated tissues. This thermal shock creates a controlled stress response that activates multiple cellular protective mechanisms, including heat shock protein expression and enhanced antioxidant enzyme production. The extreme temperature differential triggers immediate changes in nerve conduction velocity, effectively interrupting pain signal transmission while simultaneously initiating tissue-protective responses. Additionally, the rapid cooling process influences cellular metabolism, temporarily reducing oxygen consumption and metabolic waste production, which contributes to tissue preservation during acute injury phases.
The Inflammatory Cascade: A Step-by-Step Molecular View
To fully appreciate how CO₂ cryotherapy modulates inflammation, it’s essential to understand the complex molecular orchestration that characterizes the inflammatory response. This intricate biological process involves multiple cellular populations, signaling molecules, and temporal phases that work together to address tissue damage and initiate healing.
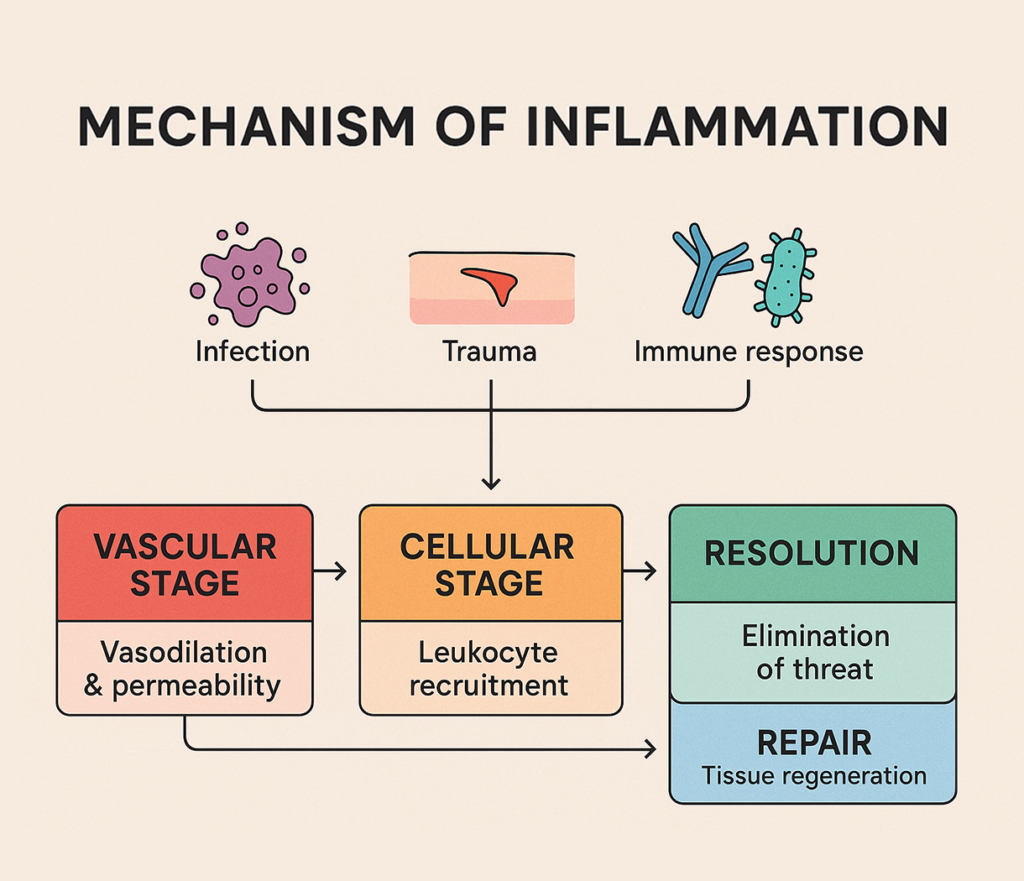
What Triggers Inflammation?
Inflammatory responses are initiated by diverse stimuli, including mechanical trauma, pathogenic invasion, chemical irritants, and metabolic stress. These triggers activate pattern recognition receptors (PRRs), particularly toll-like receptors (TLRs), which recognize damage-associated molecular patterns (DAMPs) and pathogen-associated molecular patterns (PAMPs). Upon activation, these receptors initiate intracellular signaling cascades involving nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPKs), and inflammasome complexes. These pathways ultimately lead to the production and release of pro-inflammatory mediators, including cytokines like interleukin-1β (IL-1β), interleukin-6 (IL-6), and tumor necrosis factor-alpha (TNF-α), which orchestrate the subsequent inflammatory response.
The Four Phases of Inflammation
The inflammatory process unfolds through four distinct yet overlapping phases, each characterized by specific molecular events and cellular activities. Understanding these phases provides crucial insight into how therapeutic interventions like CO₂ cryotherapy can optimize the healing process by modulating specific aspects of the inflammatory cascade.
Vascular Phase: Vasodilation and Increased Permeability
The vascular phase represents the immediate response to inflammatory stimuli, characterized by rapid changes in blood vessel behavior and endothelial function. Vasodilation occurs through the action of inflammatory mediators such as histamine, prostaglandins, and nitric oxide, which cause smooth muscle relaxation in arterioles and venules. Simultaneously, increased vascular permeability allows plasma proteins and immune cells to extravasate into tissue spaces, facilitating the delivery of immune effector molecules and cells to the site of injury. This phase is mediated by endothelial cell gap junction formation, basement membrane degradation by matrix metalloproteinases (MMPs), and altered endothelial cytoskeletal dynamics that collectively enable the characteristic signs of acute inflammation: erythema, edema, and warmth.
Cellular Phase: Neutrophil and Macrophage Infiltration
The cellular phase involves the sequential recruitment and activation of various immune cell populations, beginning with neutrophil infiltration within hours of injury onset. Neutrophils are attracted by chemotactic gradients of interleukin-8 (IL-8), complement fragments, and bacterial products, utilizing selectin-mediated rolling, integrin-mediated adhesion, and diapedesis to enter tissues. These cells release antimicrobial substances, including reactive oxygen species and neutrophil extracellular traps (NETs), while also secreting additional inflammatory mediators. Macrophages subsequently arrive, initially adopting a pro-inflammatory M1 phenotype that amplifies tissue damage through the release of cytotoxic mediators, followed by a phenotypic switch to anti-inflammatory M2 macrophages that promote tissue repair and debris clearance through phagocytosis and the secretion of growth factors.
Resolution Phase: Anti-inflammatory Cytokines and Tissue Repair
The resolution phase represents a critical transition from tissue destruction to healing, orchestrated by specialized pro-resolving mediators (SPMs) including resolvins, protectins, and maresins derived from omega-3 fatty acids. These molecules actively promote the cessation of neutrophil recruitment, enhance neutrophil apoptosis and clearance by macrophages (efferocytosis), and stimulate the production of anti-inflammatory cytokines such as interleukin-10 (IL-10) and transforming growth factor-beta (TGF-β). This phase also involves the activation of tissue-resident stem cells and the initiation of angiogenesis through vascular endothelial growth factor (VEGF) signaling. The successful completion of this phase is essential for preventing chronic inflammation and ensuring optimal tissue repair, with dysregulation leading to persistent inflammatory states that can impair healing and promote tissue damage.
Remodeling Phase: Collagen Production and Scar Formation
The remodeling phase encompasses the long-term restructuring of damaged tissues through coordinated collagen synthesis, degradation, and reorganization. Fibroblasts proliferate and differentiate into myofibroblasts under the influence of TGF-β and mechanical tension, producing type I and type III collagen fibers that provide structural support to healing tissues. Simultaneously, matrix metalloproteinases and tissue inhibitors of metalloproteinases (TIMPs) regulate collagen turnover and remodeling to optimize tissue strength and function. This phase can extend for months to years, with the balance between collagen synthesis and degradation determining final tissue architecture. Proper modulation of this phase is crucial for preventing excessive scar formation or tissue weakness, with therapeutic interventions potentially influencing long-term functional outcomes through their effects on fibroblast activity and extracellular matrix composition.
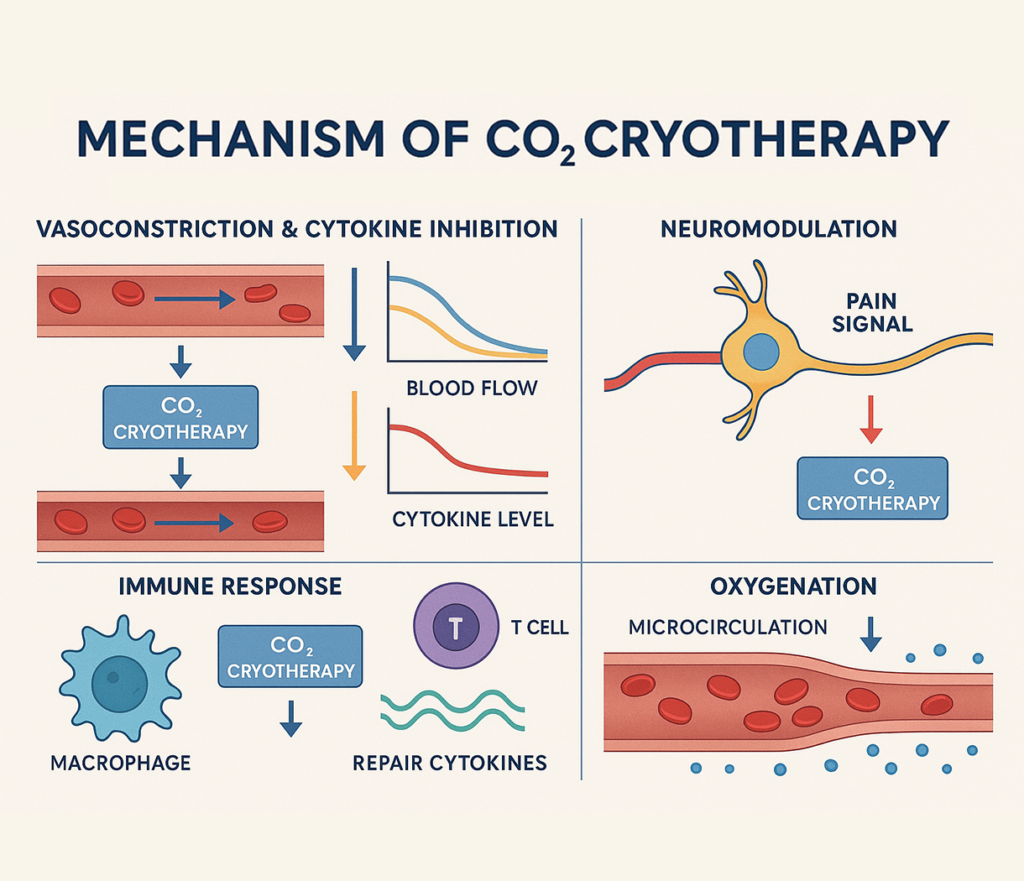
CO₂ Cryotherapy’s Effects on Inflammatory Pathways
The therapeutic potential of CO₂ cryotherapy lies in its ability to modulate multiple aspects of the inflammatory cascade simultaneously, creating synergistic effects that promote optimal healing while minimizing tissue damage. This multifaceted approach allows for precise therapeutic targeting of specific inflammatory mechanisms.
Cold-Induced Vasoconstriction and Cytokine Suppression
CO₂ cryotherapy exerts immediate vasoconstrictive effects through direct smooth muscle contraction and reduced nitric oxide availability, effectively limiting the initial vascular response to inflammatory stimuli. This vasoconstriction reduces blood flow to treated areas, limiting the delivery of inflammatory cells and mediators while simultaneously reducing tissue edema and associated pain. At the molecular level, extreme cold exposure downregulates the expression of pro-inflammatory cytokines including IL-1β, IL-6, and TNF-α through inhibition of NF-κB activation and reduced transcriptional activity. Recent studies have demonstrated that cryotherapy can reduce inflammation by decreasing proinflammatory factors like IL-1β while increasing anti-inflammatory factor IL-10, and local ice cryotherapy decreases synovial interleukin 6, interleukin 1β, and nuclear factor kappa B p65 in human knee arthritis.
Neuromodulation and Pain Reduction
The analgesic effects of CO₂ cryotherapy result from multiple neurophysiological mechanisms that interrupt pain signal transmission and modify nociceptor sensitivity. Extreme cold temperatures slow nerve conduction velocity in both myelinated and unmyelinated fibers, effectively blocking action potential propagation and reducing pain signal intensity. Additionally, cold exposure activates the gate control theory of pain modulation by stimulating large-diameter mechanoreceptors that inhibit small-diameter nociceptor transmission at the spinal cord level. The therapy also influences endogenous opioid release and modulates neurotransmitter balance in pain processing centers. These combined effects result in both immediate and prolonged analgesic benefits, with pain relief often persisting beyond the duration of tissue cooling due to altered neural plasticity and reduced inflammatory mediator production.
Immune Cell Modulation by CO₂ Cold Therapy
CO₂ cryotherapy profoundly influences immune cell behavior and phenotype, promoting a shift from pro-inflammatory to anti-inflammatory responses. Cold exposure reduces neutrophil adhesion and extravasation through altered integrin expression and endothelial cell function, limiting early inflammatory cell infiltration. Macrophage polarization is also affected, with cold therapy promoting the M2 anti-inflammatory phenotype over the M1 pro-inflammatory state through alterations in metabolic pathways and transcription factor activity. Additionally, cryotherapy influences T-cell function and regulatory T-cell (Treg) expansion, enhancing immune tolerance and resolution of inflammatory responses. The therapy also affects complement system activation and antibody production, contributing to overall immunomodulatory effects that support tissue healing while preventing excessive inflammatory damage.
Tissue Oxygenation and Microcirculation
The microcirculatory effects of CO₂ cryotherapy extend beyond simple vasoconstriction, involving complex changes in oxygen delivery, utilization, and tissue metabolism. While initial vasoconstriction reduces blood flow, subsequent reactive hyperemia following treatment cessation enhances oxygen and nutrient delivery to tissues. Cold exposure also reduces cellular oxygen consumption through decreased metabolic activity, creating a favorable oxygen balance that protects tissues from hypoxic damage during acute injury phases. Studies have shown that cryotherapy with carbon dioxide hydrate enhances immediate recovery of muscle function from neuromuscular fatigue, likely through these metabolic effects. Additionally, improved microcirculatory efficiency following cryotherapy treatment enhances waste product removal and facilitates the delivery of healing factors, contributing to accelerated tissue repair and functional recovery.
Clinical and Therapeutic Applications of CO₂ Cryotherapy
The versatility of CO₂ cryotherapy has led to its adoption across numerous clinical specialties, with applications ranging from acute injury management to chronic pain treatment. Understanding these diverse applications provides insight into the broad therapeutic potential of targeted cold therapy.
Sports Injuries and Musculoskeletal Inflammation
In sports medicine, CO₂ cryotherapy has revolutionized acute injury management and recovery protocols, offering superior outcomes compared to traditional cooling methods. The precise temperature control and rapid application capability make it ideal for treating muscle strains, ligament sprains, and joint injuries where immediate inflammation control is crucial. The therapy’s ability to penetrate deeper tissues more effectively than ice packs allows for better treatment of muscle injuries and deep tissue inflammation. Athletic trainers and sports medicine physicians utilize CO₂ cryotherapy for both acute injury treatment and preventive care, with many professional sports teams incorporating it into routine recovery protocols. The treatment’s portability and ease of application make it particularly valuable in field settings where immediate intervention can significantly impact injury severity and recovery time.
Chronic Pain and Joint Inflammation Management
For chronic pain conditions, CO₂ cryotherapy offers a non-pharmacological approach that can provide sustained relief while addressing underlying inflammatory processes. Patients with arthritis, fibromyalgia, and chronic back pain have shown significant improvement with regular cryotherapy treatments, experiencing reduced pain intensity and improved functional capacity. The treatment’s ability to modulate inflammatory cytokines makes it particularly effective for inflammatory arthropathies, where traditional treatments may have limited efficacy or significant side effects. Physical therapy clinics and pain management centers increasingly incorporate CO₂ cryotherapy into comprehensive treatment programs, often achieving better outcomes when combined with exercise therapy and manual techniques. The treatment’s drug-free nature makes it especially valuable for patients seeking alternatives to long-term pharmaceutical interventions or those who cannot tolerate conventional anti-inflammatory medications.
Veterinary and Animal Rehabilitation Use
The application of CO₂ cryotherapy in veterinary medicine has shown remarkable success, particularly in treating performance animals such as racehorses and working dogs. The therapy’s effectiveness in reducing inflammation and pain in animals mirrors its human applications, with veterinarians reporting improved outcomes in treating musculoskeletal injuries, post-surgical recovery, and chronic conditions. The precision of CO₂ delivery systems allows for safe and effective treatment of various animal sizes and anatomical regions, from small companion animals to large livestock. Animal rehabilitation centers utilize cryotherapy as part of comprehensive recovery programs, often achieving faster return to activity and improved long-term outcomes. The non-invasive nature of the treatment reduces stress on animal patients while providing effective therapeutic benefits, making it an attractive option for veterinary practitioners seeking evidence-based treatment modalities.
Comparing CO₂ Cryotherapy to Other Inflammation Treatments
Understanding how CO₂ cryotherapy compares to other anti-inflammatory treatments helps practitioners make informed decisions about optimal therapeutic approaches. This comparison reveals both the unique advantages and appropriate applications of different treatment modalities.
Traditional Ice Therapy
Traditional ice therapy, while widely accessible and commonly used, presents several limitations when compared to CO₂ cryotherapy. Ice packs typically achieve temperatures around 0°C (32°F), significantly warmer than the -78°C achievable with CO₂ systems, limiting their penetration depth and therapeutic intensity. The cooling rate with ice is also much slower, requiring 15-20 minutes to achieve therapeutic temperatures, compared to the immediate effect of CO₂ cryotherapy. Ice therapy often causes uneven cooling, skin damage from prolonged contact, and discomfort that limits patient compliance. In contrast, CO₂ cryotherapy provides consistent, controlled temperatures with precise application timing, reducing the risk of thermal injury while maximizing therapeutic benefit. The portability and ease of use of CO₂ systems also make them more practical for clinical settings and field applications where traditional ice may not be readily available or practical to use.
Pharmacological Approaches
Pharmacological anti-inflammatory treatments, including NSAIDs, corticosteroids, and disease-modifying agents, operate through systemic mechanisms that may produce side effects and contraindications limiting their use. CO₂ cryotherapy offers a localized, drug-free alternative that can achieve similar anti-inflammatory effects without systemic toxicity or drug interactions. While medications may provide longer-lasting effects, cryotherapy can be repeated as needed without concerns about dosage limits or cumulative toxicity. The immediate onset of action with cryotherapy also provides advantages over oral medications, which may take hours to achieve therapeutic levels. For patients with contraindications to anti-inflammatory medications, such as gastrointestinal disorders, cardiovascular disease, or kidney dysfunction, cryotherapy represents a safe and effective alternative. The combination of cryotherapy with reduced pharmaceutical doses may also allow for optimal inflammation control while minimizing medication-related side effects.
Scientific Evidence and Research Highlights
The growing body of scientific literature supporting CO₂ cryotherapy’s effectiveness provides a robust foundation for its clinical application. Examining key research findings helps establish evidence-based protocols and identifies areas for future investigation.
Molecular Biomarker Studies
Recent molecular studies have provided detailed insights into the biochemical mechanisms underlying cryotherapy’s anti-inflammatory effects, with biomarker analysis revealing specific pathways affected by cold exposure. Research has shown that local cryotherapy induces an intrajoint temperature decrease, which might downregulate several mediators involved in joint inflammation and destruction, including cytokines, cartilage-degrading enzymes, and proangiogenic factors. Advanced proteomics and genomics studies have identified specific gene expression changes following cryotherapy, including upregulation of anti-inflammatory genes and downregulation of inflammatory transcription factors. Metabolomics analysis has revealed alterations in cellular energy metabolism, oxidative stress markers, and lipid mediator profiles that correlate with therapeutic outcomes. These molecular insights are driving the development of personalized cryotherapy protocols based on individual biomarker profiles and genetic variants that influence treatment response.
Clinical Case Studies in Human and Animal Models
Clinical research spanning diverse populations and conditions has consistently demonstrated the efficacy of CO₂ cryotherapy across multiple outcome measures. Studies in healthy adults have shown that whole-body cryotherapy beneficially impacts systemic inflammation by lowering high-sensitivity C-reactive protein (hsCRP) levels and may also have modulating effects on fasting glucose. Controlled trials in athletic populations have documented improved recovery times, reduced pain scores, and enhanced functional outcomes compared to conventional treatments. Animal model studies have provided valuable insights into optimal treatment parameters, dosing schedules, and long-term safety profiles. Meta-analyses of clinical trials have confirmed the therapeutic benefits while identifying patient populations most likely to respond favorably to treatment. These comprehensive studies form the foundation for evidence-based clinical protocols and support the integration of cryotherapy into standard treatment algorithms.
Safety, Contraindications, and Best Practices
While CO₂ cryotherapy is generally safe when properly administered, understanding appropriate safety protocols and contraindications is essential for optimal patient outcomes and risk management.
Safety and Contraindications
CO₂ cryotherapy is contraindicated in patients with certain medical conditions that may be exacerbated by extreme cold exposure or altered circulation. Absolute contraindications include severe cardiovascular disease, uncontrolled hypertension, severe peripheral vascular disease, and cold-induced urticaria or other cold-related allergic reactions. Relative contraindications include pregnancy, severe diabetes with neuropathy, active malignancy in the treatment area, and certain autoimmune conditions. Patients with compromised sensation or circulation require careful evaluation and monitoring during treatment to prevent thermal injury. Proper training of healthcare providers is essential to ensure safe application techniques, appropriate treatment parameters, and recognition of adverse reactions. Emergency protocols should be established for rare but serious complications, and patient screening procedures must be implemented to identify contraindications before treatment initiation.
Treatment Frequency and Duration
Optimal treatment protocols for CO₂ cryotherapy vary based on the condition being treated, patient factors, and treatment goals, with most applications involving 10-15 second exposures per treatment site. Acute injuries typically benefit from multiple treatments within the first 48-72 hours, followed by gradually decreased frequency as inflammation resolves. Chronic conditions may require regular maintenance treatments, with schedules ranging from daily to weekly depending on symptom severity and patient response. Treatment duration and intensity should be adjusted based on patient tolerance, tissue response, and clinical outcomes. Practitioners must balance therapeutic effectiveness with safety considerations, avoiding overtreatment that could lead to tissue damage or adverse reactions. Documentation of treatment parameters, patient responses, and outcomes is essential for optimizing individual protocols and contributing to the evidence base for best practices.
Conclusion: CO₂ Cryotherapy as a Precision Tool for Inflammation Management
CO₂ cryotherapy marks a breakthrough in inflammation control, offering targeted treatment with minimal systemic impact. By delivering controlled cold at precise depths and durations, it modulates inflammatory pathways without relying on pharmaceuticals. Ongoing research continues to uncover the molecular mechanisms behind cryotherapy’s anti-inflammatory effects—such as reduced cytokine activity, improved microcirculation, and immune modulation—paving the way for optimized, evidence-based protocols. Its safety, reproducibility, and adaptability make CO₂ cryotherapy a valuable tool across various clinical settings, from orthopedics to neurology. As medicine embraces personalized, non-invasive therapies, cryotherapy aligns perfectly with this shift. With growing clinical validation and technological innovation, CO₂ cryotherapy is positioned to become a mainstay in precision inflammation management—delivering measurable relief and better patient outcomes with fewer side effects.
FAQs About CO₂ Cryotherapy and Inflammation
CO₂ cryotherapy provides immediate anti-inflammatory effects, with noticeable reduction in swelling and pain often occurring within minutes of treatment. The molecular changes begin immediately upon cold exposure, with cytokine levels decreasing within hours and continued improvement over 24-48 hours following treatment.
When properly administered by trained professionals, CO₂ cryotherapy can be safely used daily for acute conditions. However, treatment frequency should be individualized based on the specific condition, patient response, and clinical judgment. Chronic conditions may benefit from less frequent maintenance treatments.
While CO₂ cryotherapy can be highly effective for managing inflammation, it should not replace prescribed medications without physician consultation. Many patients find that cryotherapy allows for reduced medication dosages or provides an effective alternative for those who cannot tolerate traditional anti-inflammatory drugs.
CO₂ cryotherapy achieves much colder temperatures (-78°C vs 0°C), provides more rapid and uniform cooling, offers precise temperature control, and eliminates the risk of frostbite associated with prolonged ice application. These factors result in superior therapeutic outcomes and improved patient experience.
Current research indicates that properly administered CO₂ cryotherapy has minimal long-term side effects. The treatment is non-invasive and drug-free, reducing the risk of cumulative toxicity associated with long-term medication use. However, long-term studies continue to monitor safety profiles as the technology becomes more widely adopted.



