Introduction: Where Science Meets Cold Therapy
The intersection of advanced cryotherapy technology and neuromodulation represents one of the most promising frontiers in contemporary pain management and rehabilitation medicine. As healthcare providers increasingly seek evidence-based, non-pharmaceutical interventions for pain relief and tissue recovery, the synergy between carbon dioxide cryotherapy and neuromodulatory mechanisms has emerged as a compelling therapeutic approach.
Rise of CO₂ Cryotherapy in Modern Medicine
Contemporary medical practice has witnessed a remarkable transformation in the application of cryotherapy, particularly through the integration of carbon dioxide delivery systems. Unlike traditional ice application or whole-body cryotherapy chambers, CO₂ cryotherapy offers precise, targeted cooling with exceptional temperature control and consistent therapeutic outcomes. The precision afforded by modern CO₂ delivery systems allows clinicians to achieve specific temperature targets while maintaining optimal treatment durations, fundamentally changing how we approach localized tissue cooling in clinical settings.
Why Neuromodulation Is Gaining Attention
Neuromodulation has gained significant traction in pain management due to its ability to modify neural activity without permanent tissue damage or systemic side effects. This therapeutic approach targets specific neural pathways, offering clinicians the ability to modulate pain perception, reduce inflammation, and promote tissue healing through controlled neural interventions. The growing body of research supporting neuromodulatory techniques has positioned them as essential components of comprehensive pain management protocols.
Что такое криотерапия CO₂?
Understanding the fundamental principles of CO₂ cryotherapy requires examining both its mechanical properties and physiological effects. This therapeutic modality represents a sophisticated evolution of traditional cryotherapy, offering enhanced precision, safety, and clinical efficacy through advanced carbon dioxide delivery systems.
Definition and History of CO₂-Based Cryotherapy
CO₂ cryotherapy involves the controlled application of carbon dioxide in its solid or liquid state to achieve therapeutic tissue cooling. The technique emerged from dermatological applications in the mid-20th century, where practitioners utilized carbon dioxide snow for various skin conditions. Modern CO₂ cryotherapy systems have evolved significantly, incorporating sophisticated temperature monitoring, precise delivery mechanisms, and safety protocols that ensure consistent therapeutic outcomes while minimizing adverse effects.
How It Differs from Whole-Body and Liquid Nitrogen Cryotherapy
The primary distinction between CO₂ cryotherapy and other cryotherapeutic modalities lies in its precision, safety profile, and mechanism of action. While whole-body cryotherapy exposes the entire body to extreme cold temperatures, CO₂ cryotherapy allows for targeted application to specific anatomical regions. Compared to liquid nitrogen systems, CO₂ cryotherapy operates at more moderate temperatures with enhanced safety margins, reducing the risk of frostbite or tissue damage while maintaining therapeutic efficacy.
Common Devices: Local CO₂ Cryotherapy Machines for Humans
Современные аппараты для криотерапии CO₂ incorporate sophisticated engineering to deliver precise, controlled cooling through specialized applicators and delivery systems. These devices typically feature adjustable temperature controls, varied applicator sizes for different anatomical regions, and integrated safety mechanisms to prevent tissue damage. The technology enables practitioners to achieve consistent therapeutic temperatures while monitoring treatment parameters in real-time, ensuring optimal patient safety and treatment outcomes.
Key Mechanisms: Thermal Shock, Vasoconstriction, and Tissue Cooling
The therapeutic effects of CO₂ cryotherapy result from multiple physiological mechanisms triggered by controlled tissue cooling. Thermal shock initiates immediate vasoconstriction, reducing local blood flow and inflammatory mediator delivery to treated tissues. Subsequent tissue cooling modulates cellular metabolism, reduces enzymatic activity, and creates an environment conducive to pain reduction and tissue healing. These mechanisms work synergistically to produce both immediate analgesic effects and longer-term therapeutic benefits.
Understanding Neuromodulation
Neuromodulation encompasses a broad spectrum of therapeutic interventions designed to alter neural activity through targeted stimulation or inhibition of specific neural pathways. This field has evolved from basic electrical stimulation techniques to sophisticated approaches that can selectively modulate different aspects of neural function, offering unprecedented precision in pain management and neurological rehabilitation.
What Is Neuromodulation?
Neuromodulation refers to the therapeutic alteration of nerve activity through controlled delivery of electrical, chemical, or physical stimuli to specific neural targets. This approach modifies the electrical patterns of neural networks, influencing how the nervous system processes and transmits information. Unlike pharmacological interventions that affect the entire system, neuromodulation can be precisely targeted to specific neural circuits, allowing for customized therapeutic approaches based on individual patient needs and pathophysiology.
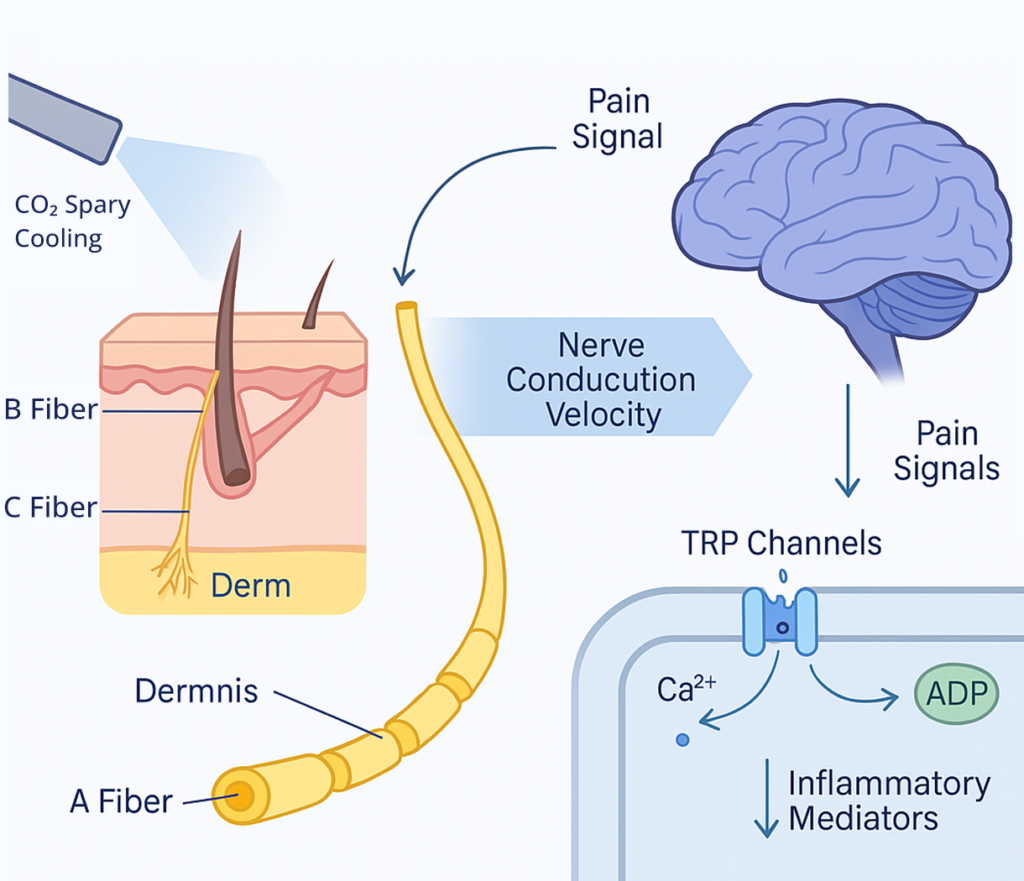
Key Terms: Nociceptors, C-Fibers, and Aδ-Fibers
Understanding neuromodulation requires familiarity with essential neural components involved in pain processing. Nociceptors are specialized sensory receptors that detect potentially harmful stimuli and convert them into electrical signals. C-fibers are unmyelinated nerve fibers that transmit dull, aching pain sensations, while Aδ-fibers are lightly myelinated fibers responsible for sharp, acute pain perception. These neural structures represent key targets for neuromodulatory interventions, each responding differently to various therapeutic approaches.
Role in Pain Signaling and Brain-Body Communication
Pain signaling involves complex neural networks that transmit information from peripheral tissues to the central nervous system. This process involves multiple levels of modulation, from peripheral nociceptor activation to spinal cord processing and brain interpretation. Neuromodulation can intervene at various points along this pathway, altering signal transmission, processing, and perception. Understanding these communication pathways is essential for developing targeted therapeutic interventions that can effectively modulate pain experience.
Neuromodulation vs. Nerve Block: What’s the Difference?
While both neuromodulation and nerve blocks target neural function, their mechanisms and outcomes differ significantly. Nerve blocks involve the temporary or permanent interruption of neural transmission through chemical agents or physical interventions, effectively silencing neural activity. Neuromodulation, conversely, modifies neural activity patterns without completely blocking transmission, allowing for more nuanced control over neural function. This distinction is crucial for understanding when each approach is most appropriate and effective.
How CO₂ Cryotherapy Affects Neuromodulation
The interaction between CO₂ cryotherapy and neuromodulation represents a sophisticated therapeutic relationship that leverages the unique properties of controlled cooling to influence neural function. This synergy creates opportunities for precise, targeted interventions that can modulate pain perception, reduce inflammation, and promote tissue healing through well-defined physiological mechanisms.
Cooling and Nerve Conduction Velocity
Controlled tissue cooling through CO₂ cryotherapy directly affects nerve conduction velocity (NCV), a fundamental parameter of neural function. Research demonstrates that cryotherapy significantly reduces nerve conduction velocity, with this reduction being associated with increased pain threshold and pain tolerance. Temperature reduction slows the movement of ions across neural membranes, decreasing the speed at which electrical signals travel along nerve fibers. This physiological change creates a window of reduced neural excitability, allowing for therapeutic intervention while maintaining neural integrity.
Local Analgesic Effects via Sensory Receptor Inhibition
CO₂ cryotherapy produces immediate analgesic effects through direct inhibition of peripheral sensory receptors and neural terminals. The cooling effect temporarily reduces the sensitivity of nociceptors and other sensory receptors, creating a localized analgesic zone. This mechanism is particularly effective for acute pain conditions, post-surgical discomfort, and inflammatory pain states. The duration and intensity of these effects can be controlled through treatment parameters, allowing for customized therapeutic protocols.
Modulating Chronic Pain Through Peripheral Nervous System Pathways
Chronic pain often involves sensitized peripheral nerves that keep sending pain signals, even without tissue damage. CO₂ cryotherapy helps break this cycle by briefly resetting nerve activity. It also reduces inflammation linked to pain sensitivity. This method shows promise for neuropathic pain, complex regional pain syndrome, and chronic musculoskeletal issues.
Evidence-Based Mechanisms: TRP Channels, ATP Depletion, and Ion Channel Modulation
The neuromodulatory effects of CO₂ cryotherapy involve multiple molecular mechanisms that have been extensively studied in contemporary research. Transient receptor potential (TRP) channels, particularly TRPV1, TRPM8, and TRPA1, are key targets for cold-induced neuromodulation, as these channels are expressed in primary afferent nociceptors and contribute to pain detection. Cooling affects ion channel function, reduces ATP availability for neural transmission, and modulates calcium influx patterns that are essential for neural signal propagation. These mechanisms work collectively to create comprehensive neuromodulatory effects.
Short-Term Relief vs. Long-Term Neuroplastic Adaptation
CO₂ cryotherapy produces both immediate therapeutic effects and longer-term neuroplastic adaptations that can provide sustained pain relief. Short-term benefits include immediate analgesic effects, reduced inflammation, and decreased neural excitability. Long-term adaptations involve changes in neural pathway sensitivity, reduced central sensitization, and improved pain processing efficiency. Understanding this temporal relationship is crucial for developing treatment protocols that maximize both immediate relief and sustained therapeutic benefits.
Scientific Evidence and Clinical Research
The growing body of scientific evidence supporting CO₂ cryotherapy and neuromodulation reflects rigorous research efforts to understand and validate these therapeutic approaches. Contemporary research spans multiple disciplines, from basic neuroscience to clinical applications, providing comprehensive insights into mechanisms, efficacy, and safety profiles.
Studies Supporting CO₂ Cryotherapy for Neuromuscular Recovery
Recent research demonstrates that cryotherapy with carbon dioxide hydrate enhances immediate recovery of muscle function from neuromuscular fatigue, providing compelling evidence for its therapeutic efficacy in athletic and rehabilitation settings. Studies have shown that CO₂ cryotherapy can accelerate recovery processes, reduce muscle fatigue, and improve neuromuscular performance metrics. These findings support the integration of CO₂ cryotherapy into comprehensive recovery protocols for athletes and patients undergoing rehabilitation.
CO₂ Cryotherapy in Sports Medicine and Rehabilitation
Sports medicine applications of CO₂ cryotherapy have demonstrated significant benefits for injury prevention, acute injury management, and recovery optimization. Research indicates that localized CO₂ cryotherapy can reduce exercise-induced muscle damage, accelerate recovery from training sessions, and improve overall athletic performance. The precision of CO₂ delivery systems makes them particularly valuable for treating specific anatomical regions commonly affected by sports-related injuries, such as joints, tendons, and muscle groups.
Literature on Neuromodulation and Cold Therapy Synergy
The synergistic relationship between cold therapy and neuromodulation has been extensively documented in pain management literature. Studies demonstrate that combining cryotherapy with other neuromodulatory techniques can enhance therapeutic outcomes compared to individual interventions. Research on hyperbaric CO₂ cryotherapy has shown significant pain reduction in elderly patients with various rheumatic diseases, supporting the clinical efficacy of these combined approaches in diverse patient populations.
Safety and Tolerability: What the Data Says
Clinical safety data for CO₂ cryotherapy demonstrate excellent tolerability profiles when proper protocols are followed. Industry trends emphasize enhanced accuracy and stability in temperature control, improving treatment effectiveness and safety. Adverse events are typically mild and transient, including temporary skin redness, mild discomfort during treatment, and occasional skin sensitivity. The safety profile of CO₂ cryotherapy compares favorably to other cryotherapeutic modalities, with reduced risk of tissue damage due to more controlled temperature delivery.
CO₂ Cryotherapy vs. Other Neuromodulatory Techniques
Comparing CO₂ cryotherapy to other neuromodulatory approaches provides valuable insights into optimal treatment selection and protocol development. Each modality offers unique advantages and limitations, making understanding their comparative effectiveness essential for clinical decision-making and patient care optimization.
Electrical Nerve Stimulation (TENS) vs. Cold-Based Neuromodulation
Transcutaneous electrical nerve stimulation (TENS) and CO₂ cryotherapy represent fundamentally different approaches to neuromodulation, each with distinct mechanisms and clinical applications. TENS uses electrical impulses to stimulate nerve fibers, while CO₂ cryotherapy achieves neuromodulation through controlled cooling. TENS offers advantages in terms of treatment duration flexibility and patient control, while CO₂ cryotherapy provides more immediate analgesic effects and reduced treatment complexity. The choice between these modalities depends on specific clinical indications, patient preferences, and treatment goals.
Comparing Cryotherapy to Pulsed Electromagnetic Fields (PEMF)
Pulsed electromagnetic field (PEMF) therapy and CO₂ cryotherapy both offer non-invasive neuromodulatory approaches with distinct mechanisms of action. PEMF therapy uses electromagnetic fields to influence cellular function and neural activity, while CO₂ cryotherapy achieves similar goals through controlled cooling. Both modalities demonstrate efficacy in pain reduction and tissue healing, but they differ in treatment protocols, duration of effects, and patient experience. Understanding these differences helps clinicians select the most appropriate intervention for specific clinical scenarios.
When to Choose Cryotherapy: Use Case Scenarios
Optimal selection of CO₂ cryotherapy depends on specific clinical indicators, patient characteristics, and treatment objectives. Acute inflammatory conditions, post-surgical pain management, and localized musculoskeletal injuries represent ideal applications for CO₂ cryotherapy. The technique is particularly valuable when rapid onset of analgesic effects is desired, when precise targeting of specific anatomical regions is required, or when other modalities have proven insufficient. Patient factors such as skin sensitivity, circulation status, and treatment tolerance also influence selection criteria.
Complementary Use: Combining Cryotherapy with Other Modalities
The integration of CO₂ cryotherapy with other therapeutic modalities can enhance treatment outcomes through synergistic mechanisms. Combining cryotherapy with physical therapy, manual therapy, or other neuromodulatory techniques can provide comprehensive pain management approaches that address multiple aspects of patient care. Sequential or concurrent use of different modalities requires careful consideration of timing, dosing, and potential interactions. These combination approaches often provide superior outcomes compared to single-modality treatments.
Clinical Applications and Use Cases
The clinical applications of CO₂ cryotherapy and neuromodulation span diverse medical specialties and patient populations, reflecting the versatility and effectiveness of these therapeutic approaches. Understanding specific use cases helps clinicians optimize treatment protocols and patient selection for maximum therapeutic benefit.

Treating Sciatica, Neuropathy, and Nerve Entrapment Syndromes
Nerve-related pain conditions represent prime candidates for CO₂ cryotherapy interventions due to the direct relationship between neural function and temperature modulation. Sciatica, characterized by radiating pain along the sciatic nerve distribution, can benefit from targeted cooling that reduces nerve inflammation and modulates pain transmission. Peripheral neuropathies, including diabetic neuropathy and chemotherapy-induced neuropathy, respond well to cryotherapy through reduced neural excitability and improved pain threshold. Nerve entrapment syndromes such as carpal tunnel syndrome benefit from the anti-inflammatory and analgesic effects of controlled cooling.
Post-Surgical Pain and Inflammation Control
Post-operative pain management represents a critical application for CO₂ cryotherapy, offering effective analgesia while potentially reducing opioid requirements. The technique provides immediate pain relief through neural modulation while simultaneously reducing inflammatory responses that contribute to post-surgical discomfort. Surgical site application of CO₂ cryotherapy can minimize tissue swelling, reduce inflammatory mediator release, and create optimal conditions for tissue healing. This application is particularly valuable in orthopedic, plastic surgery, and general surgical procedures.
Chronic Conditions: Fibromyalgia, CRPS, and Rheumatic Pain
Chronic pain conditions that involve central sensitization and persistent inflammatory states can benefit significantly from CO₂ cryotherapy interventions. Fibromyalgia patients often experience reduced pain intensity and improved quality of life through targeted cooling of trigger points and tender areas. Complex regional pain syndrome (CRPS) responds well to cryotherapy through interruption of sympathetic nervous system hyperactivity and reduction of inflammatory cascades. Rheumatic conditions including arthritis benefit from the anti-inflammatory and analgesic properties of controlled cooling.
Enhancing Recovery in Sports and Occupational Therapy
Athletic and occupational therapy applications of CO₂ cryotherapy focus on optimizing recovery processes, preventing injury, and enhancing performance outcomes. Post-exercise application can reduce muscle fatigue, accelerate recovery, and prepare athletes for subsequent training sessions. Occupational therapy integration helps workers manage repetitive strain injuries, reduce work-related pain, and maintain productivity. The precision of CO₂ delivery systems makes them particularly valuable for targeting specific muscle groups or anatomical regions commonly affected by sports or occupational activities.
Conclusion: Unlocking New Frontiers in Pain Management
The convergence of CO₂ cryotherapy and neuromodulation represents a paradigm shift in pain management, offering evidence-based, precise, and effective interventions that address the complex nature of pain perception and tissue healing. This therapeutic approach has demonstrated remarkable versatility across diverse clinical applications while maintaining excellent safety profiles.
Summary of Neuromodulatory Mechanisms
The neuromodulatory effects of CO₂ cryotherapy operate through multiple interconnected mechanisms that collectively produce comprehensive therapeutic outcomes. Temperature-induced changes in nerve conduction velocity, modulation of TRP channels, and alteration of inflammatory pathways work synergistically to reduce pain perception and promote tissue healing. These mechanisms provide both immediate analgesic effects and longer-term neuroplastic adaptations that can provide sustained therapeutic benefits. Understanding these mechanisms enables clinicians to optimize treatment protocols and maximize patient outcomes.
CO₂ Cryotherapy as a Future-Proof Solution
The evolution of CO₂ cryotherapy technology continues to advance, with improvements in precision, safety, and efficacy that position it as a cornerstone of modern pain management. The industry-wide shift towards more precise, efficient, and safer cryotherapy technologies reflects the growing recognition of its therapeutic potential. Future developments may include enhanced temperature control systems, improved delivery mechanisms, and integration with other therapeutic modalities. These advances will likely expand the clinical applications and effectiveness of CO₂ cryotherapy across diverse patient populations.
Who Can Benefit Most from This Synergy?
The greatest beneficiaries of CO₂ cryotherapy and neuromodulation include patients with chronic pain conditions, athletes seeking performance optimization, post-surgical patients requiring effective pain management, and individuals with inflammatory conditions. Healthcare providers in pain management, sports medicine, rehabilitation, and primary care settings can integrate these techniques into comprehensive treatment protocols. The versatility and safety of CO₂ cryotherapy make it suitable for diverse patient populations, from young athletes to elderly patients with chronic conditions.
Часто задаваемые вопросы (FAQ)
CO₂ cryotherapy works by rapidly cooling tissues to approximately -78°C, which slows cellular metabolism, reduces enzyme activity, and modulates ion channel function. This creates an environment that reduces inflammatory mediator release while temporarily altering neural excitability.
Treatment sessions typically last 10-15 seconds per application site, with the exact duration depending on the specific condition being treated, tissue depth, and individual patient factors. Multiple applications may be performed during a single session.
While generally safe, CO₂ cryotherapy may not be suitable for patients with certain conditions including severe circulation problems, cold sensitivity disorders, or certain skin conditions. A thorough medical evaluation should precede treatment.
Many patients experience immediate pain relief during and immediately after treatment, with effects lasting several hours to days. Cumulative benefits may develop over multiple treatment sessions.
Yes, CO₂ cryotherapy can be effectively combined with physical therapy, manual therapy, and other neuromodulatory techniques. Combination approaches often provide superior outcomes compared to single-modality treatments.
Conditions that typically respond well include acute injuries, post-surgical pain, arthritis, nerve entrapment syndromes, and chronic pain conditions with inflammatory components.